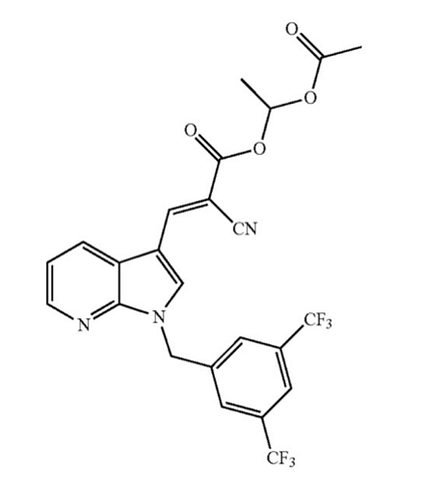
PP405
(CAS: unknown)
Discussion of molecular identity
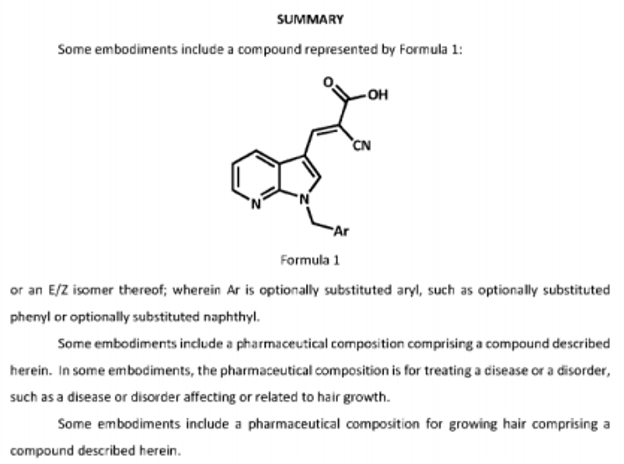
The structure of PP405 has not generally been disclosed, hence there is speculation about the molecular structure of the compound. The following section hence discusses likely
candidates for the identity of PP405.
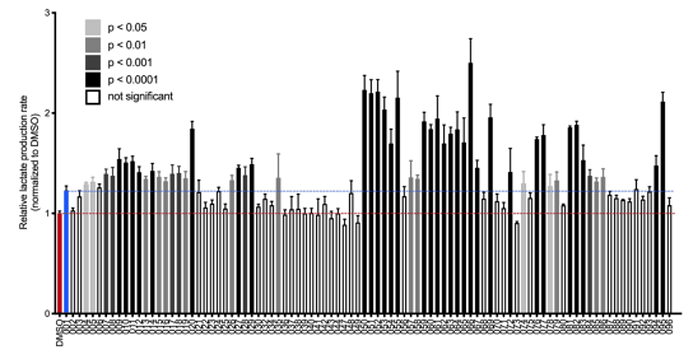
There is one publication which describes the synthesis of new derivatives of the group mitochondrial pyruvate carrier inhibitors, which PP405 is very likely a part of.1 In this publication several similar compounds are synthesized and tested for their ability to regrow hair in mice and their in vitro ability to lead to higher production rates of lactate. The following graph shows the ability of the compounds to increase lactate production, which is an indicator of how effective the mitochondrial pyruvate carrier is blocked.
According to the data, the 10 best producers of lactate are the compounds:
- JXL 066
- JXL 050
- JXL 052
- JXL 051
- JXL 095
- JXL 055
- JXL 054
- JXL 069
- JXL 061
- and JXL 059
All these belong to the group that have either OH or Oet at the carbonyl group, indicating that PP405 is likely also in this group. The most active compounds also all have a 2,4- bis(trifluormethyl)phenyl group as aryl group, hence PP405 likely also has this as well. Also,
the additional nitrogen atom in the 6 membered ringed of the fused heterocycles (7-aza- indole) showed very slightly better activity vs the indole derivative.
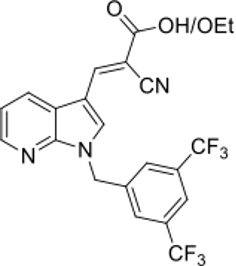
The reason why many believe that PP405 could be JXL-069 is because in the 2024 patent application from Pelage Pharmaceuticals the following structure is listed on page 1 of the document.
Section of patent application WO2024145369A1
Together with the results from the published study, which show that compounds with the aryl substituent with the 2,4-trifluoromethyl have a higher ability to produce lactate compared to other patterns, this makes JXL-069 a likely candidate to be PP405.
Claims that PP405 is JXL-082 (the ethyl ester) seem unlikely, as the main focus of the patent clearly does lie on derivatives of the aryl group and not in the ester moiety, which is only briefly mentioned.
More likely on the other side is that PP405 (if it is not JXL-069) would have an alternative aryl moiety, which was not described in the original publication but emphasized in the patent. More speculations would need further experimental evidence however.
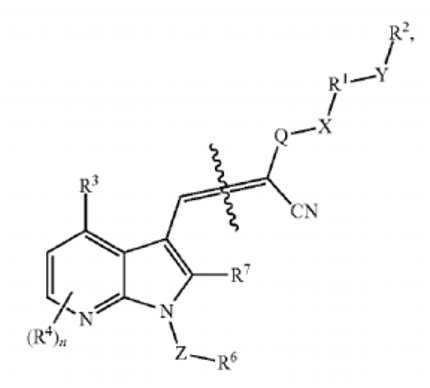
In addition to the previous patent, another patent application of pelage (US20240327400A1) mostly deals with ways to modify the existing basic structure into a prodrug at the
carboxylate group. This seems to indicate that the previous substances could not have optimal pharmacological properties for example. The described structures have the following modifications:
Section from patent US20240327400A1
There are multiple possible modifications of the carbonyl group. Some of the possible entities are covered by a later section of the patent where most of the structures are modified esters, which create possible cleavage sides for esterases or non-enzymatic breakdown which will lead to the liberation of the carboxylate compound, which is theorized to be the active compound itself, even though the initial experiments suggested that esters can be effective as well.
The most common structural motifs shown in this patent are designed for instability to quickly decompose into JXL-069 like PP20 and PP30, but other compounds of the patent also follow the same basic idea. The principle behind PP20 and PP30 is a combination of an ester (PP20) or a carbonate (PP30) with a short C1-C2 Linker. When ester or carbonate are cleaved, an instable hemiacetal-like intermediate arises, which then cleaves into JXL-069 and formaldehyde (PP20) or acetaldehyde (PP30). Formaldehyde is more toxic, however in the small concentrations expected from this release it will likely not lead to significant tissue damage.
Structure of PP30 (from US20240327400A1)
History
PP405 is under development by Pelage Pharmaceuticals and was patented in 2024. Clinical phase 1 and 2 (NCT06393452) trials are currently conducted but no official results have been posted. The company has released selected preliminary data from the phase 1 trial, stating that:
“31% of those treated with PP405 exhibited a greater than 20% increase in hair density, compared to 0% of patients responding in the placebo group. ”
which shows a potential clinical application in hair loss treatment.
Mechanism of Action
PP405 is part of the class of mitochondrial pyruvate carriers. The ability of the hair follicles to maintain this cycle depends on the presence of hair follicle stem cells (HFSCs). The HFSCs are typically inactive but can quickly “wake up” and actively divide when a new hair growth cycle is initiated. When HFSCs fail to activate, hair loss occurs. The activation of HFSCs can be achieved by stimulating the activity of lactate dehydrogenase (LDH), the enzyme catalysing the reduction of pyruvate to lactate. When the mitochondrial pyruvate carrier (MCP) is pharmacologically inhibited, pyruvate in the cytoplasm cannot enter the mitochondria and instead is converted to other metabolites, such as lactate by lactate dehydrogenase (LDH), which results in the increase of LDH activity and the activation of HFSCs. Hence, inhibition of the MCP by PP405 leads to an increase in LDH activity and stimulation of hair follicle stem cells, which results in hair growth.
Formulation
In the sole phase 2 clinical trial, the formulation is listed as 0.05 % topical gel, while in the animal study, it was described as 20 µM suspended lotion (roughly 10 mg/kg = 0,001%). It is not clear what exactly the constituents of gel are, but the lotion is referenced as “transdermal PLO Ultramax gel” .
Commercial formulations of products targeting the hair follicle are usually not supplied as gel, as gel hinders liquid from entering the follicles. Instead, less viscous liquids are used based in alcohols such as ethanol and propylene glycol as co-solvents to facilitate dissolving the API and diffusion though cells membranes.
Why a gel is used in the phase 2 trial is not clear, this may be to the early developmental stage of the drug.
Transdermal systems should specifically not be used, as the compound should exclusively be active in the hair follicles and not diffuse into the bloodstream, as systemic MCP inhibition could cause metabolic problems.
References
Liu X, Flores AA, Situ L, Gu W, Ding H, Christofk HR, Lowry WE, Jung ME.
Development of Novel Mitochondrial Pyruvate Carrier Inhibitors to Treat Hair Loss. J Med Chem. 2021;64:2046-2063. doi: 10.1021/acs.jmedchem.0c01570
PP405: A Novel Hair Loss Treatment Candidate Under Investigation